45 what is an open label study
Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced non ... This multicenter, open-label, Phase II study randomized 110 patients to receive nimotuzumab plus chemotherapy (nimotuzumab group) or chemotherapy alone (control group), and comprised concomitant, maintenance, and follow-up phases. ... This multicenter, randomized, open-label, active-controlled, prospective Phase II study was designed to ... Open label study | definition of open label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
Understanding Clinical Trial Terminology: What is an Open Label ... Jun 24, 2019 · Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an extended period of time.
What is an open label study
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study. A Phase 1 Open Label Study of the Safety and Tolerability of Oral ICM20 ... This is a Phase 1 open label study to access the safety and tolerability of ICM20 alone and in combination with benznidazole. Study Design. Go to Top of Page Study Description Study Design Arms and Interventions Outcome Measures Eligibility Criteria Contacts and Locations More Information. National Center for Biotechnology Information National Center for Biotechnology Information
What is an open label study. Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years). What is an Open-Label Trial? - Get Essay Writing Help at $10/Page An open-label trial is a clinical trial conducted in a way that allows subjects and researchers to know which treatments are being used. National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. Open-label Definition & Meaning - Merriam-Webster open-label: [adjective] being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject — compare double-blind, single-blind.
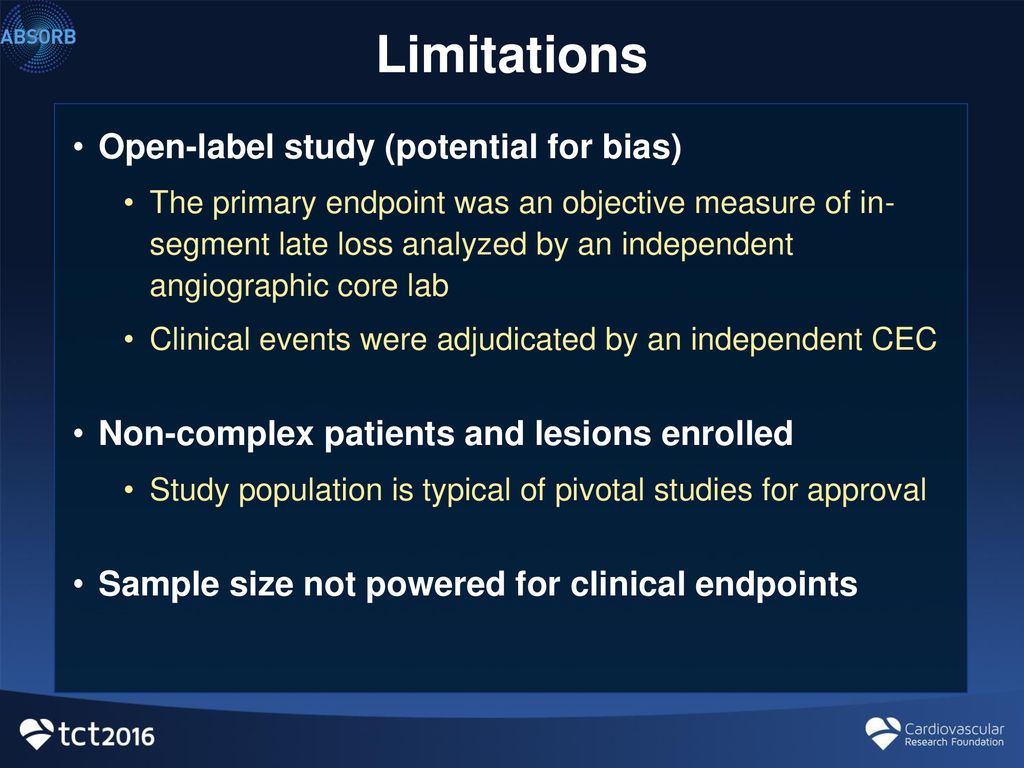
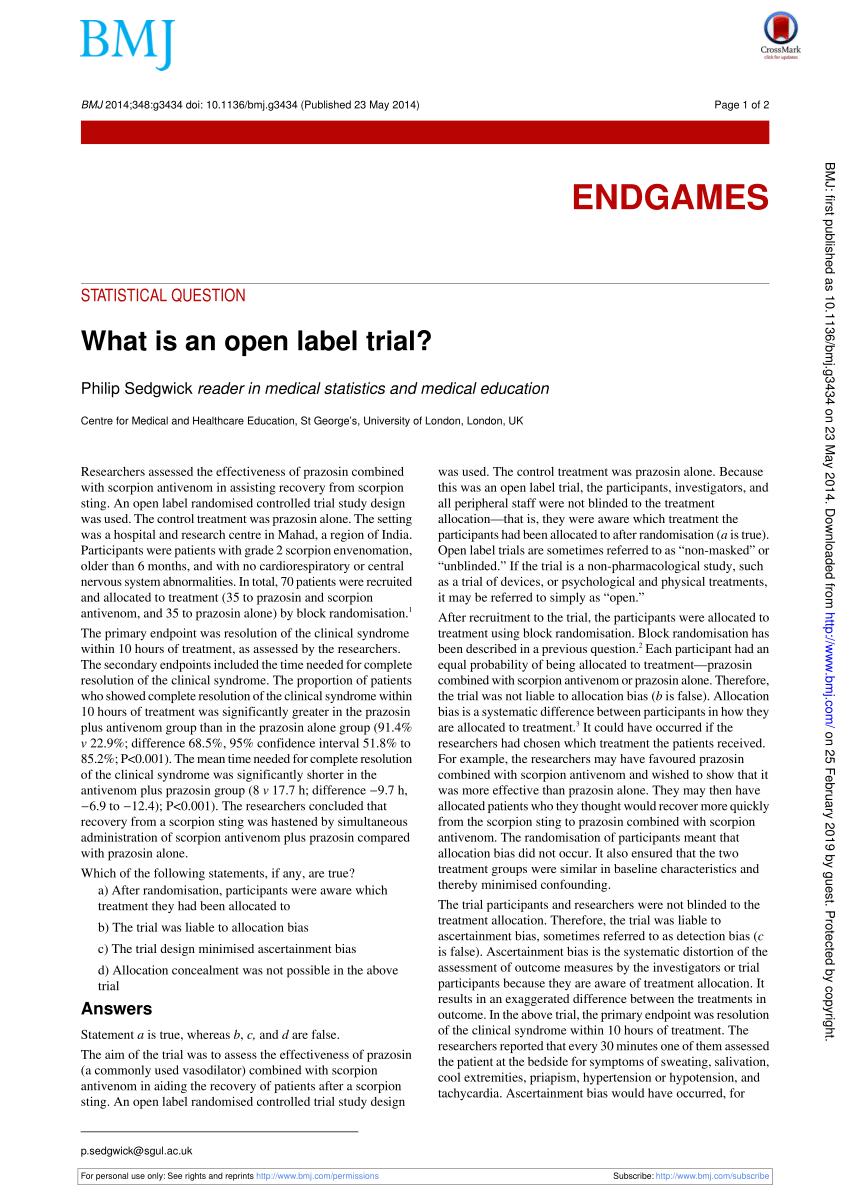
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. (PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants... Evolocumab Benefits Accrue With Longer Follow-up: FOURIER OLE Long-term lipid lowering with evolocumab (Repatha) further reduces cardiovascular events, including CV death, without a safety signal, according to results from the FOURIER open-label extension ... Reducing bias in open-label trials where blinded outcome assessment is ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment allocation. Research has suggested that these trials should use blinded outcome assessment to avoid bias in estimated treatment effects [6-10]. Blinded outcome assessment is often ...
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers and the participants to reduce bias. What is an open label trial? | The BMJ May 23, 2014 · Open label trials are sometimes referred to as “non-masked” or “unblinded.” If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as “open.” After recruitment to the trial, the participants were allocated to treatment using block randomisation. Open-Label Extension Study of ASTORIA - Full Text View - ClinicalTrials.gov Open-Label Extension Study of ASTORIA. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Open-Label Trial | NIH - HIV.gov A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
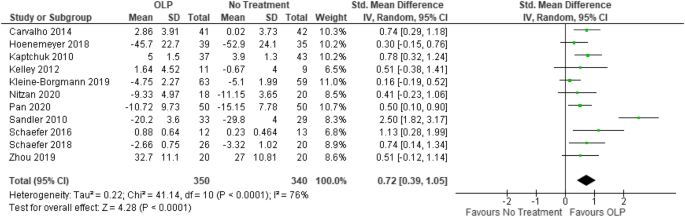
Can Open-Label Placebos Be Effective Medical Treatments? The effectiveness of open-label placebos. Dr. Jeremy Howick, a clinical epidemiologist, reviewed five research studies involving a total of 260 patients and found that open-label placebos often yield the same positive results as reflected by the placebo effect. These studies involved a variety of conditions, including irritable bowel syndrome ...
An Open-Label Study Evaluating the Safety, Behavioral, and ... Table 1. Demographic characteristics of the study population [insert here] Study Design This study utilized a single-dose (0.5 mg/kg), open-label design to investigate the safety, tolerability, and behavioral outcomes of treatment with low-dose ketamine in ten children with ADNP syndrome.
Medical Definition of Open-label - MedicineNet Reviewed on Privacy & Trust Info Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving.
What is an Open-Label Clinical Trial? - News-Medical.net Clinical trials are vital for the design and development of safe drugs, treatments, and medical interventions and bringing them to market. Often, the idea for a clinical trial starts in the laboratory. Researchers will test new drugs and treatments in animal models, with the most promising being considered for clinical trials. There are four phases...
Open-label versus double-blind placebo treatment in irritable bowel ... The study also examines possible genetic and psychological predictors of OLP and seeks to better understand participants' experiences with OLP and DBP through a series of extensive interviews with a randomly selected subgroup. ... and episodic migraine, have demonstrated that individuals receiving open-label placebo (OLP) can still experience ...
Open-Label Trial - an overview | ScienceDirect Topics In open label trials, both the study participant (and caregiver where applicable) and the investigator are aware of the treatment provided to study participants. A common use for this design is in an extension trial, which immediately follows a randomized controlled trial, and is used for gathering safety data in the same subjects.
Open Label Extension Study Definition | Law Insider Examples of Open Label Extension Study in a sentence. Rigel shall conduct such Open Label Extension Study in a good scientific manner, in compliance in all material respects with all Applicable Laws and all applicable portions of the Transition Plan.. AZ shall be solely responsible for the development of the Products in the Field in the Territory, and shall assume responsibility to fund the On ...
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Open-label study - definition of Open-label study by The Free Dictionary 2. the acquisition of knowledge or skill in a particular branch of learning, science, or art: the study of law. 3. Often, studies. a student's work at school or college: to pursue one's studies. 4. something studied or to be studied. 5. a detailed investigation and analysis of a subject, phenomenon, etc.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized
National Center for Biotechnology Information National Center for Biotechnology Information
A Phase 1 Open Label Study of the Safety and Tolerability of Oral ICM20 ... This is a Phase 1 open label study to access the safety and tolerability of ICM20 alone and in combination with benznidazole. Study Design. Go to Top of Page Study Description Study Design Arms and Interventions Outcome Measures Eligibility Criteria Contacts and Locations More Information.
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study.




















![PDF] ZINC HYALURONATE IN THE TREATMENT OF DIABETIC FOOT ...](https://d3i71xaburhd42.cloudfront.net/fc5a851ceb2630c27c4ef0786beda8efd5be5fae/3-Table1-1.png)







![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)

![PDF] VISUALIZE: a 24-week,open-label study using nasal ...](https://d3i71xaburhd42.cloudfront.net/d19226694b9d9ac9df8adaeeeb13fbd2dd62ba9c/2-Table1-1.png)






Post a Comment for "45 what is an open label study"