39 which of the following is not an fda required component of a food packaging label?
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ... Federal Register :: Food Labeling: Revision of the Nutrition and ... Furthermore, we intend to continue a variety of activities such as conduct and report on existing and planned food labeling research; to develop education initiatives at the national and local levels; to build labeling education exchanges; and to integrate food labeling education into existing programs (e.g., USDA-school-based nutrition ...
Food labeling: MedlinePlus Medical Encyclopedia Many foods are not required to have information on them. They are exempt from food labeling. These include: Airline foods Bulk food that is not resold Food service vendors (such as mall cookie vendors, sidewalk vendors, and vending machines) Hospital cafeterias Medical foods Flavor extracts Food colors Food produced by small businesses
Which of the following is not an fda required component of a food packaging label?
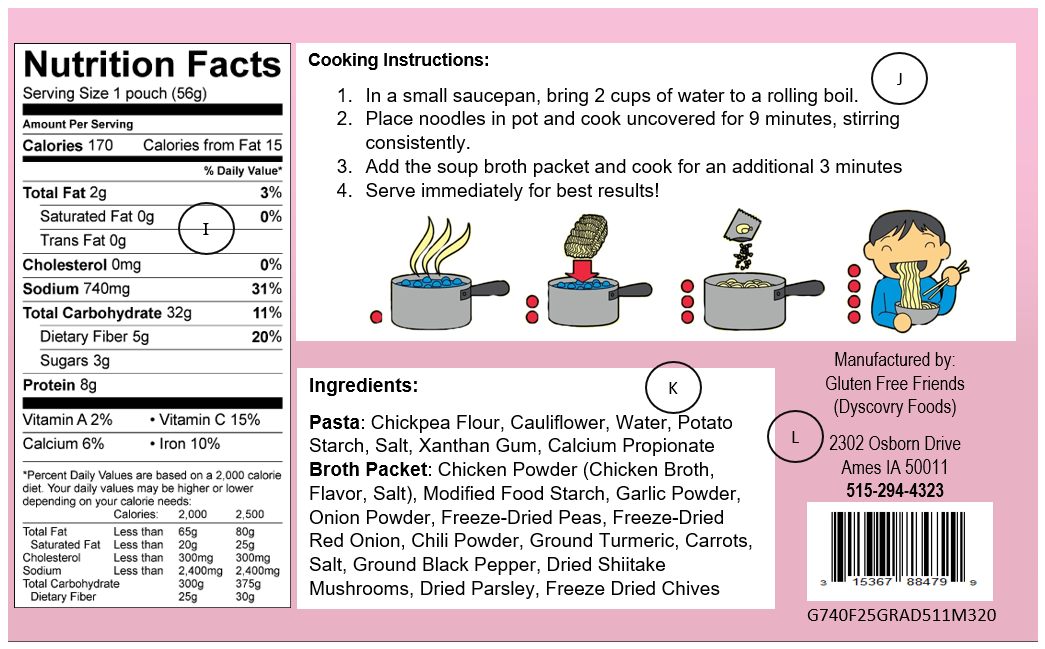
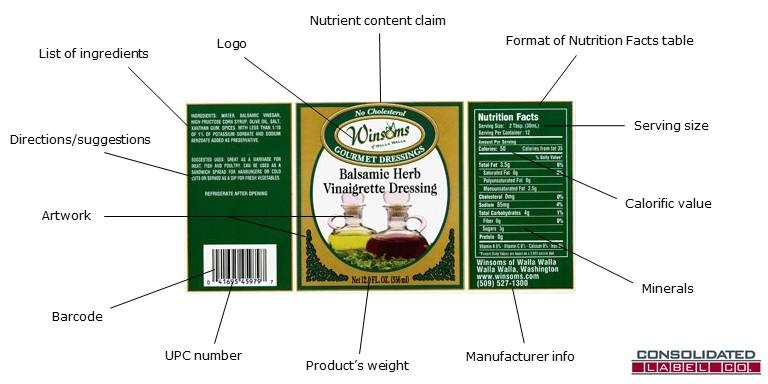
FDA Food Product Labeling & Packaging Requirements | ESHA Research Required Packaging Elements and Placement. Statement of identity, or name of the food. Net quantity of contents, or amount of product. Nutrition Facts Label. Ingredient Statement. Allergen Declaration. Name and address of the manufacturer, packer, or distributor. Additional Information. Nutrient Content Claims. Chapter 5: Food Labels Flashcards - Quizlet This fact remains true whether or not the milk proclaims it. What are the eight common allergens that have to be listed on food labels? 1) milk 2) eggs 3) fish 4) shellfish 5) tree nuts (cashews, walnuts, almonds, etc.) 6) peanuts 7) wheat 8) soybeans What are the three main reasons that food labels are so important? FDA's Packaging & Labeling Guidelines for CBD - TEKLYNX Make sure that the product is held under appropriate conditions wherein the packaging and labels are not adversely affected. Packaging must be in conditions that ensure no mixup, contamination, or deterioration of components, dietary supplements, packaging, and labels. Manufacturers are required to include an e-signature on their product labels.
Which of the following is not an fda required component of a food packaging label?. A Basic Guide to IQ, OQ, PQ in FDA-Regulated Industries As a component of quality assurance, equipment validation is absolutely critical to producing consistent, high-quality products. One of the key sets of protocols within equipment validation is Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). This guide offers a clear and simple explanation of ... Food and Drug Administration (FDA) Regulations - FedEx Under the FDA Food Safety Modernization Act (FSMA), the VQIP was established to enable the expedited importation of any FDA-regulated human and animal foods into the United States. It is a new, voluntary, fee-based program for importers. Applicants must maintain a high level of compliance over the safety and security of their supply chains to ... Rules and Standards for Food Processing — Food Law USDA's Operating Procedures for Egg Processing (9 CFR §§590.504 to 590.575) FDA's HACCP for Juice and Seafood Processing (21 CFR Parts 120 & 123) USDA's SSOP and HACCP for Meat and Poultry Processing (9 CFR Parts 416 & 417) FDA's Food Safety Plan for all other Food Processors (21 U.S.C. §305g; 21 CFR Part 117 Subpart C) eCFR :: 21 CFR Part 101 -- Food Labeling (9) The declaration of nutrient and food component content shall be on the basis of food as packaged or purchased with the exception of raw fish covered under § 101.42 (see 101.44), packaged single-ingredient products that consist of fish or game meat as provided for in paragraph (j)(11) of this section, and of foods that are packed or canned in water, brine, or oil but whose liquid packing medium is not customarily consumed (e.g., canned fish, maraschino cherries, pickled fruits, and ...
FDA Compliant, Food Grade and Food Safe | ISM - Industrial Spec Food, beverage and drinking water contact materials must not contaminate food nor make food equipment difficult to clean and sanitize. NSF tests and certifies a variety of plastic materials and resins for food contact end use. Plastics resins suitable for evaluation by the NSF for food contact certification include. Coatings; Silicone; Nylon (PA) Nutrition- Chapter 2 Mastering health Flashcards | Quizlet The FDA requires the manufacturer's, packer's, or distributor's name and address to be listed on a food package. Which of the following is NOT an FDA required component of a food packaging label? A. net weight or volume of the product B. nutrient claim C. list of ingredients D. Nutrition Facts Pane Answer: B Code of Federal Regulations Title 21 - Food and Drug Administration (1) Wearing outer garments suitable to the operation in a manner that protects against the contamination of food, food-contact surfaces, or food-packaging materials. (2) Maintaining adequate... All about Food Labels - HealthCheckSystems Lean. Less than 10 grams of fat, 4.5 g of saturated fat, and 95 mg of cholesterol per (100 gram) serving of meat, poultry or seafood. Light (fat) 50% or less of the fat than in the comparison food (ex: 50% less fat than our regular cheese) Light (calories) 1/3 fewer calories than the comparison food. High-Fiber.
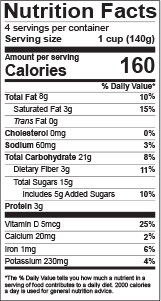
Solved Which of the following is not true for the | Chegg.com Which of the following is not true for food labels? a. the ingredients in ingredients list are listed from the least predominant to most predominant (by weight) in the product b. the ingredients list can help you determine whether a bread contains more whole grain flour than refined flour c. by law, manufacturers must list major allergens eCFR :: 21 CFR Part 801 -- Labeling (a) The appropriate FDA Center Director may grant an exception or alternative to any provision listed in paragraph (f) of this section and not explicitly required by statute, for specified lots, batches, or other units of a medical device, if the Center Director determines that compliance with such labeling requirement could adversely affect the safety, effectiveness, or availability of such devices that are or will be included in the Strategic National Stockpile. What foods are required to have food labels by law? Labels must bear the required Nutrition Facts Chart. FDA requires food labels to bear a Nutrition Facts Chart. Nutrition Facts Charts contain information such as a serving size, the number of calories the product contains, and the amount of fat, sodium, protein, and other ingredients in the product. Which foods are required to have FDA food ... Nutrition Facts Labeling — FDA Reader Nutrient Components: The nutrition information label must include some mandatory components (i.e. calories, fat) and may include other voluntary components (vitamin A). No other declarations of nutrition information is allowed on the label, other than those listed below: Mandatory Nutrient Components. Calories "Fat" or "Total Fat" Saturated Fat. Trans Fat
Food Ingredients & Packaging | FDA Irradiation of Food & Packaging FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria in...
National Drug Codes Explained: What You Need to Know The labeler code is assigned by the U.S. Food and Drug Administration (FDA), while the product and package code are assigned by the labeler. For billing or other purposes, such as with the Centers for Medicare & Medicaid Services (CMS), an NDC may also be arranged in an 11-digit format with leading zeros, if needed.
Nutrition Labels 101: What's Required? What's Optional? - Medallion Labs Vitamins A and C will no longer be required on the FDA's Nutrition Facts labels (though manufacturers may still include them if they choose), while Vitamin D and Potassium will now be required. The percent of the daily value is expressed in 2% increments from 2-10% of the daily value; in 5% increments from 10 to 50% of the daily value; and in 10% increments if the level is above 50%.
Food Product Dating | Food Safety and Inspection Service Except for infant formula, product dating is not required by Federal regulations. [1] For meat, poultry, and egg products under the jurisdiction of the Food Safety and Inspection Service (FSIS), dates may be voluntarily applied provided they are labeled in a manner that is truthful and not misleading and in compliance with FSIS regulations. [2]
Packaging, Labeling, Transporting, Storing — Food Law Indirect food additives (e.g., packaging) may be only used under conditions of good manufacturing practice; that is, the quantity 1) does not exceed the amount reasonably required to accomplish the intended effect in the food, 2) shall not exceed any prescribed limitations, 3) shall not be intended to accomplish any physical or technical effect in the food except as permitted by regulation, and 4) the article that contacts food shall be of a purity suitable for its intended use.
List of ingredients and allergens on food labels - Canadian Food ... Wax coating compounds, their components and other protective edible coatings are not required to be shown on the labels of prepackaged fresh fruits or fresh vegetables as an ingredient or a component [B.01.008 (3) (a), FDR ]. Apples, turnips and cucumbers are examples of fruits and vegetables that have wax coatings.
FREEDOM OF INFORMATION SUMMARY - animaldrugsatfda.fda.gov effective. Effectiveness, target animal safety and hu man food safety data (other than tissue residue data) are not required for approval of an ANADA. If bioequivalence is demonstrated through a clinical endpoint study in a food producing animal, then a tissue residue study to establish the withdrawal period for the generic product is also ...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
CFR - Code of Federal Regulations Title 21 (b) Records shall be maintained for all components, drug product containers, closures, and labeling for at least 1 year after the expiration date or, in the case of certain OTC drug products...
How to Read Food Labels - Introduction to Food Label Claims Common phrases included on food product labels are "use by," "sell by," "best by," and "better if used by.". Labels may utilize a familiar date listing or may use the Julian Calendar, where each number of the year is assigned a number from 1-365. Providing a date on a food is not required and is voluntary.
Packaging and labelling | Food Standards Agency Some foods are exempt from the need to display an ingredient list, for example: fresh fruit and vegetables, carbonated water and foods consisting of a single ingredient etc. For further information...
FDA's Packaging & Labeling Guidelines for CBD - TEKLYNX Make sure that the product is held under appropriate conditions wherein the packaging and labels are not adversely affected. Packaging must be in conditions that ensure no mixup, contamination, or deterioration of components, dietary supplements, packaging, and labels. Manufacturers are required to include an e-signature on their product labels.
Chapter 5: Food Labels Flashcards - Quizlet This fact remains true whether or not the milk proclaims it. What are the eight common allergens that have to be listed on food labels? 1) milk 2) eggs 3) fish 4) shellfish 5) tree nuts (cashews, walnuts, almonds, etc.) 6) peanuts 7) wheat 8) soybeans What are the three main reasons that food labels are so important?
FDA Food Product Labeling & Packaging Requirements | ESHA Research Required Packaging Elements and Placement. Statement of identity, or name of the food. Net quantity of contents, or amount of product. Nutrition Facts Label. Ingredient Statement. Allergen Declaration. Name and address of the manufacturer, packer, or distributor. Additional Information. Nutrient Content Claims.




![The 6-Step Product Development Process [Explainer] - Shopify ...](https://cdn.shopify.com/s/files/1/0070/7032/files/new-product-development-process.jpg?v=1600652722)























Post a Comment for "39 which of the following is not an fda required component of a food packaging label?"